

These are generally different types of metals or other chemical compounds. The electrons flow from one electrode called the anode (or negative electrode) to another electrode called the cathode (the positive electrode). To produce a flow of electrons, you need to have somewhere for the electrons to flow from, and somewhere for the electrons to flow to. To understand this properly, we need to have a closer look at the cell's components, and how they are put together. In an electrochemical cell, electrons are produced by a chemical reaction that happens at one electrode (more about electrodes below!) and then they flow over to the other electrode where they are used up.
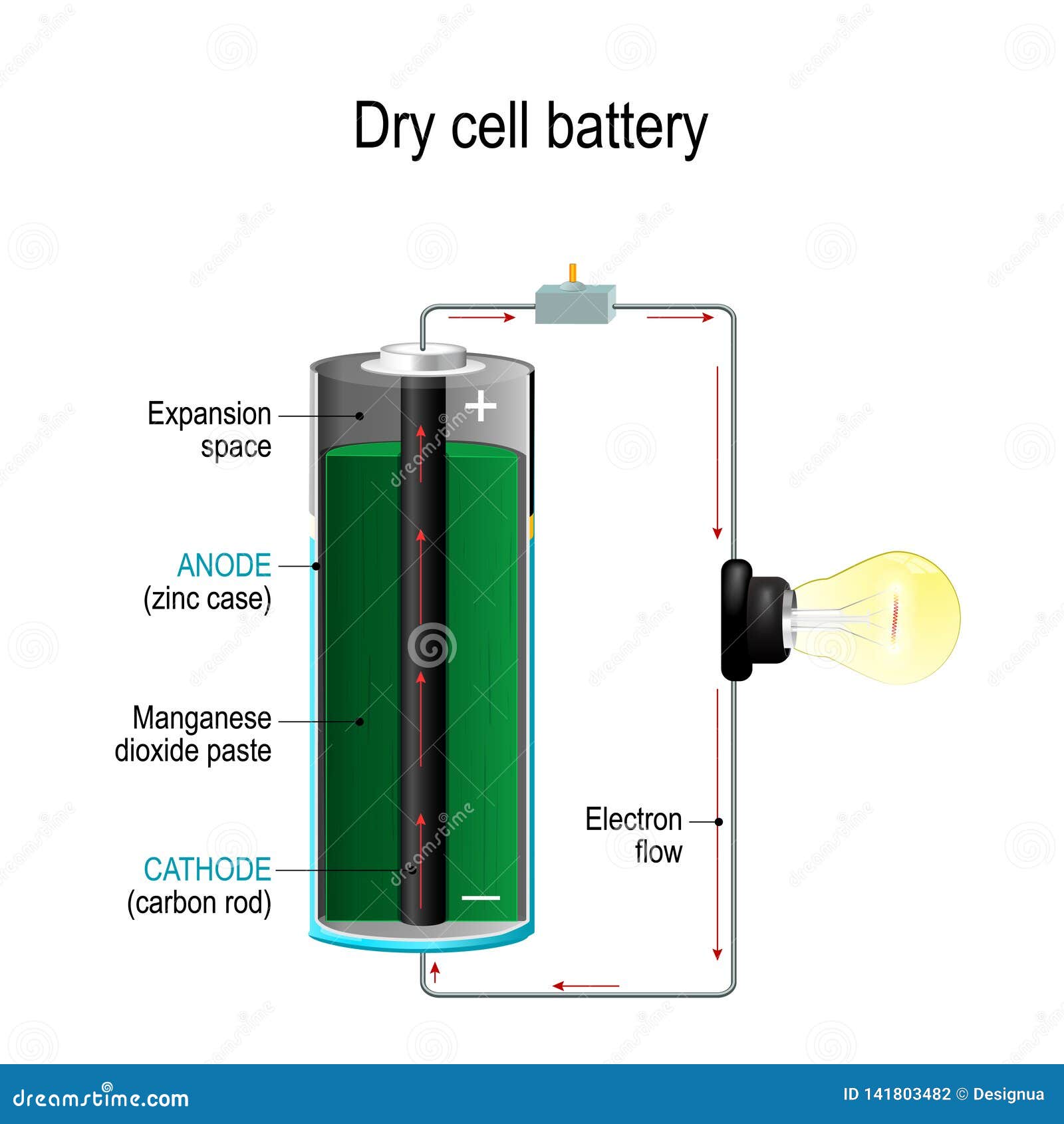
Most simply, electricity is a type of energy produced by the flow of electrons. So where does an electrochemical cell get its electricity from? To answer this question, we need to know what electricity is. Each electrochemical cell consists of two electrodes separated by an electrolyte.

A battery can be made up of one or several (like in Volta's original pile) electrochemical cells. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. So what exactly was happening with those layers of zinc and silver, and indeed, the twitching frogs’ legs?Ī battery is a device that stores chemical energy, and converts it to electricity. pure and simple electricity caused by the contact of metals, could have produced so much excitement. I myself, joking aside, am amazed how my old and new discoveries of. It was a pretty big deal (Napoleon was fairly impressed!) and his invention earned him sustained recognition in the honour of the ‘volt’ (a measure of electric potential) being named after him. He described his findings in a letter to Joseph Banks, then president of the Royal Society of London, in 1800. Volta also found that by using different metals in the pile, the amount of voltage could be increased. Image source: Luigi Chiesa / Wikimedia Commons. Alessandro Volta’s battery: a pile of zinc and silver sheets interspersed with cloth or paper soaked in saltwater. He experimented with stacks of layers of silver and zinc interspersed with layers of cloth or paper soaked in saltwater, and found that an electric current did in fact flow through a wire applied to both ends of the pile. Volta, while initially impressed with Galvani’s findings, came to believe that the electric current came from the two different types of metal (the hooks on which the frogs were hanging and the different metal of the probe) and was merely being transmitted through, not from, the frogs’ tissues. Image source: Luigi Galvani / Wikimedia Commons. He thought this response was caused by ‘animal electricity’ from within the frog. Luigi Galvani found that the legs of frogs suspended on brass hooks would twitch when prodded with a probe made of another type of metal. He believed that this was caused by electricity from within the frogs’ tissues, and called it ‘animal electricity’. In 1780, Galvani had shown that the legs of frogs hanging on iron or brass hooks would twitch when touched with a probe of some other type of metal. The invention of the battery as we know it is credited to the Italian scientist Alessandro Volta, who put together the first battery to prove a point to another Italian scientist, Luigi Galvani. Archaeologists believe these were not actually batteries but were used primarily for religious ceremonies. Back in 150 BC in Mesopotamia, the Parthian culture used a device known as the Baghdad battery, made of copper and iron electrodes with vinegar or citric acid.
#Anode and cathode in lead acid battery portable#
All those portable devices we’re so dependent on would be so limited! We’d only be able to take our laptops and phones as far as the reach of their cables, making that new running app you just downloaded onto your phone fairly useless.


 0 kommentar(er)
0 kommentar(er)
